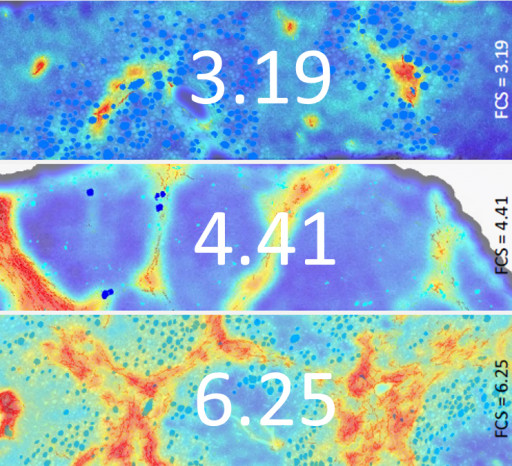
FibroNest's AI scores Fibrosis Severity in Liver Biopsies
Liver Biopsies in three different stages of fibrosis severity are automatically scored by Fibronest with reproducibility and detection thresholds that exceed current methods
The QUID-NASH Research Consortium (Paris, France) and PharmaNest, Inc. (Princeton, USA) announced today that they have entered a strategic partnership that will support the development and validation of a virtual liver biopsy (non-invasive) and artificial intelligence (AI)-powered tool for the diagnosis and staging of NASH (Non-Alcoholic Steatohepatitis) in patients with type 2 diabetes (T2D).
The clinical study involves 600 type 2 diabetic patients who are receiving a liver biopsy as part of standard care. The data collected (imaging, deep clinico-biological phenotyping, different omic approaches, extracellular vesicles and immune blood cell profiling) will be analyzed with the Swiss Institute of Bioinformatics (SIB) and Bichat biostatistical teams through comprehensive and deep bioinformatics and biostatistics.
Through its cloud-based, Digital Pathology platform FibroNest, PharmaNest will generate single-fiber and single-nuclei high-content quantitative histological datasets from each digital biopsy image to generate continuous scores that quantify the phenotypes of fibrosis and stage the severity of fibrosis and disease activity of NASH. This dataset will enrich the data collected and enable the consortium to discover novel histological phenotypes of NASH in T2D patients.
"The quantitative FibroNest method resolves the limitation of the current categorical and semi-quantitative methods and paradigms used of the histological assessment of liver biopsies," says Prof. Valerie Paradis, Chair of the Pathology Department at Beaujon Hospital and leader of the INSERM team.
"PharmaNest is excited to enable the QUID NASH consortium to enter the research field of Pathomic-Fusion and provide a robust and automated method for the quantification of the fibrosis," says Mathieu Petitjean, Ph.D., CEO of PharmaNest, Inc.
"The addition of FibroNest's Digital Pathology and Artificial intelligence capabilities to the QUID-NASH consortium will enhance performance of our upcoming virtual biopsy tests and generate significant upside for the investigation and understanding of NASH in T2D patients," says Pr. Dominique Valla, Principal Investigator and founding director of the French Reference Center for Vascular Diseases of the Liver
About QUID-NASH
The QUID-NASH research program is a clinical research consortium that includes Assistance Publique-Hôpitaux de Paris, Universities Paris-Descartes and Paris-Diderot, Commissariat à l'Energie Atomique (CEA), Laboratoires Servier and BioPredictive which operate under the leadership of the National Institute of Health and Medical Research (INSERM) of France. Clinical sites and teams include diabetology in Lariboisière and Cochin hospitals, Hepatology in Beaujon and Cochin, Imaging in Necker Beaujon and Physics for Medecine Paris.
About PharmaNest
PharmaNest is a Digital Pathology and Artificial Intelligence company focused on the development and validation of novel histological standards for the quantification of Inflammation and fibrosis. Its multivendor platform, FibroNest, is delivered worldwide via the cloud and used in multiple pre-clinical and clinical studies across several fibrotic conditions.
For more information about FibroNest and PharmaNest, visit www.pharmanest.com and www.fibronest.com
Media Contact
Mathieu M. Petitjean, Ph.D.
CEO, PharmaNest
Press Release Service by Newswire.com
Original Source: The QUID-NASH Research Consortium (Paris, France) and PharmaNest, Inc. (Princeton, USA) Enter a Strategic Partnership for the Study of NASH