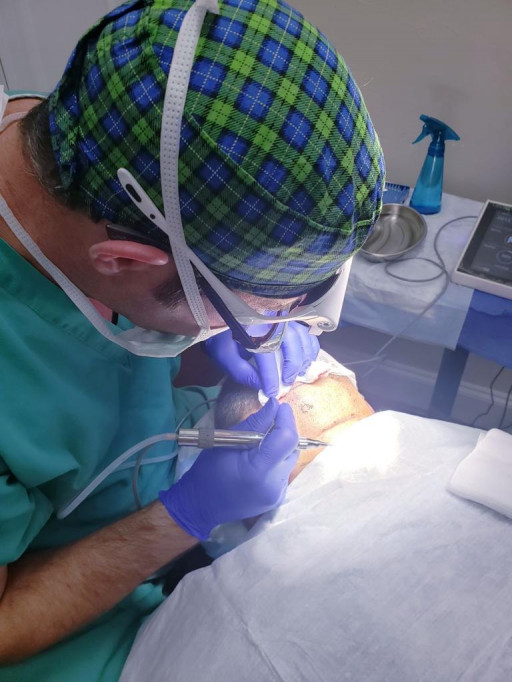
Dr. Smith With Trivellini Hair Transplant Tool
Dr. Jesse Smith performs follicular unit extraction with Trivellini hand piece.
Jesse E. Smith, MD, FACS, facial plastic surgeon, announced the purchase of revolutionary hair transplantation equipment from Trivellini Inc. The follicular unit extraction device offers superior graft survival rates, creating a fuller head of hair in less time.
"It's all about the survival rate, and that starts with the extraction device," says Dr. Smith. "[The competition] is basically just a spinning drill press. Whereas the Trivellini uses vibration, oscillation, and less rotation, slowly driving the punch into the scalp, so less trauma."
Trivellini includes multiple unique features for a more successful extraction process, and ultimately, a better hair transplant overall. To start with, the Flared Ring Punch angles the hair follicle away from the cutting edge, reducing the risk of transection and follicular unit loss during extraction.
And Trivellini's "Smart React" technology automatically recognizes when the punch is pressed against the skin and starts the extraction.
The multiphasic settings allow Dr. Smith to dial in exactly how much rotation, oscillation, and vibration he wants at each stage as the punch first moves through epidermis, then into softer tissue, and finally to dislodge the follicular unit.
The results:
- Less trauma
- Fewer transections
- Greater graft survival rate
- Fuller hair
Jesse E. Smith, MD, FACS is a facial plastic surgeon in Dallas-Fort Worth and Colleyville, Texas. Dr. Smith is dual board-certified in Facial Plastic and Reconstructive Surgery as well as Head and Neck Surgery. His specialties include rhinoplasty, hair transplant, facelift procedures, cancer and reconstruction, and minimally invasive.
Press Release Service by Newswire.com
Original Source: Dr. Jesse Smith Purchases Trivellini Hair Transplant Tech